orange book pharmacy definition
It is the publication of Approved Drug Products. Familiarly known as the Orange Guide this title is an essential reference work for all those involved in the manufacture and distribution of medicines in Europe.

The 2017 Orange And Green Guides Mhra Inspectorate
Risk is inherent in everything we do to deliver high-quality services.
. CSA 7395 7245 7498 1590 7025 7034 7011 7431 7031 7012 DEA SUBSTANCE NUMBER SCH NARC OTHER NAMES 4-Methyl-25-dimethoxyamphetamine I N DOM STP. Book Cover TBA e Product summary. Orange-Book-Standard issued in 2009 by the German Federal Court of Justice on the interaction between patent law and standards.
Rules and Guidance for Pharmaceutical Manufacturers and Distributors commonly known as the Orange Guide brings together all the main European and UK directives regulations and legislation relating to the manufacture and distribution of medicines. The 2022 edition of Rules and Guidance for Pharmaceutical Manufacturers and Distributors the Orange. FDA orange book The official name of FDAs orange book is Approved Drug Products with Therapeutic Equivalence Evaluations.
Rechtsprechung Standard essential patents FRAND commitments and competition law. Trusted for more than 120 years RED. Before understanding different drug ratings it is necessary.
We make every effort to prevent errors and discrepancies in the Approved Drug Products data files. Food and Drug AdministrationFDA has approved as both safe and effective. 1 Generic substitution laws are state specific and many require use of the Orange Book.
Freedom of information proves as a search engine for any drug approval process. Originally this book was published in October 1980 with orange cover and thus the name orange book. The Orange Book is an important publication published by the FDA that serves as the gold standard reference for generic drug substitution.
The Orange Book is a list of drugs and pharmaceuticals that the US. Effective and meaningful risk management in government. 1 approved prescription drug products with therapeutic equivalence evaluations.
A The maximum reasonable fee for pharmaceuticals and pharmacy services rendered after January 1 2004 is 100 of the reimbursement prescribed in the relevant Medi-Cal payment system including the Medi-Cal professional fee for dispensing. The full publication title is Approved Drug Products with Therapeutic Equivalence Evaluations but it is commonly known as the Orange Book. The orange book is published annually and the 2015 edition is 35th edition of orange book1 It is freely available for.
_ GITAM Institute of Pharmacy. The Orange Book downloadable data files are updated monthly. Information and translations of Orange-Book in the most comprehensive dictionary definitions resource on.
Red Book 30th Edition 2015 Aap Ebooks Pharmacys fundamental reference. First published in 1971 the original Orange Guide contained British Good Manufacturing Practice and was entitled Guide to Good Pharmaceutical Manufacturing Practice. Orange Book a local area networking protocol based on the Cambridge Ring and one of the UK Coloured Book protocols.
FDAs Approved Drug Products with Therapeutic Equivalence Evaluations Orange Book identifies drug products approved on the basis of safety and effectiveness. CONCLUSION The Orange Book thus gives basic information related to the drug approval process. Builds on the previous Orange Book to help improve risk management further and to embed this as a routine part of how we operate.
Good manufacturing practice GMP is the minimum standard that a medicines manufacturer must meet in their production processes. Public sector organisations cannot be risk averse and be successful. Meet the requirements of the marketing authorisation.
Our online pharmacy sells quality products in the USA Canada and around the world. Rather than parts 1 or 2 of the orange book as discussed in section 11 must be submitted to the orange book. An analysis under particular consideration of the German Orange-Book-Standard-decisions Torsten Körber.
100812 BMCP 215 SURAT. It is widely accepted as the authoritative source for determining therapeutic equivalence among multisource drug products. The Orange Book is composed of four parts.
It should be read and used in conjunction with the other publications such as the Green Book which provides specific advice on appraisal and evaluation. Also a list of drugs covered by an insurance company. Formally known as Approved Drug Products with Therapeutic Equivalence Evaluations the orange book lists drugs which are not only safe but also effective for human use.
It is compiled by the UK drug regulatory body MHRA and brings together the European and UK guidance documents and information on legislation. An introduction a how to use section the drug product lists appendices and a patent and exclusivity information addendum. Sumanta Mondal_MPhar m 1 th Sem.
Although it is commonly called the Orange Book its formal name is Approved Drug Products with Therapeutic Equivalence Evaluations. The Orange Book does not include drugs. 2 approved over-the-counter OTC drug products for those drugs that.
Red Book Pharmacy Definition. The orange book consist of five main sections. Handbook of Directives and Permitted Conventions for the English Bridge Union.
The orange book is a list of generic drugs approved by FDA. The Orange Book formally titled Approved Drug Products With Therapeutic Equivalence Evaluations is a comprehensive list of approved drug products published by the FDA. Not much more than 30 pages in length this voluntary guide was an aid to manufacturers to understand the needs of the regulatory authoritys requirements for the manufacture of.
Published 29 May 2013 Last updated 23.

The Orange Guide Medicinescomplete

The Introduction Of An Orange Book

Investigational New Drug Orange Book Understanding On 505 B 2 A
Emerging Vectors Of Narratology

The Introduction Of An Orange Book

The Introduction Of An Orange Book

Pharmaceutical Press Rules And Guidance For Pharmaceutical Manufacturers And Distributors 2022 The Mhra Orange Guide

Therapeutic Equivalence Codes Effects Substitution Video Lesson Transcript Study Com

Schizophrenia Recognition And Management The Pharmaceutical Journal

The Introduction Of An Orange Book

The 2017 Orange And Green Guides Mhra Inspectorate

The Introduction Of An Orange Book
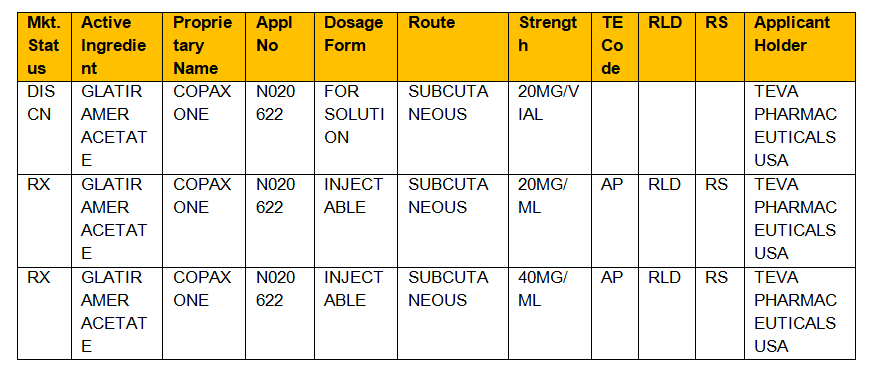
Orange Book And Its Applications Legal Advantage
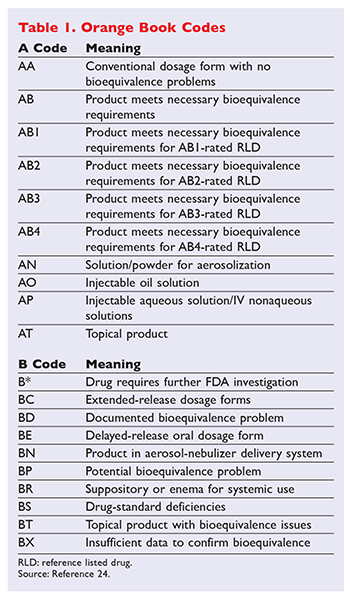
Insights Into Effective Generic Substitution

The Introduction Of An Orange Book

Learn How To Become A Pharmacist The Steps To This Career Can Vary By State See The Details Pharmacist Education Pharmacist Pharmacy School
Regulatory 101 Drug Name Modifiers Definition Categories Generics And Capa Ivt
/doctor-826e0c116cd549d98e327f1184c622d9.jpg)